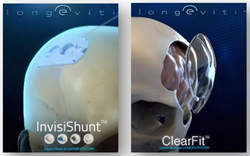

Engineered Medical Systems, Longeviti Neuro Solutions and The LaunchPort™ announced today that they have entered into an agreement to place new, low temperature, low toxicity hydrogen peroxide sterilization capacity at The LaunchPort™/EMS facility in Port Covington. The systems are being procured by Longeviti to support the Maryland production of their Low-profile Intracranial Devices (L.I.D.) platform. The Longeviti L.I.D. Platform has led to several innovative FDA cleared products thus far, the ClearFit and InvisiShunt, and more are in development. The systems will be operated by Engineered Medical Systems within EMS’s regulated medical manufacturing facility at LaunchPort™. These new systems are expected to have completed initial validations by the end of Q1 2020.
Image: https://www.prweb.com
