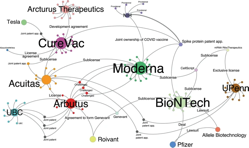
The COVID-19 pandemic has had a substantial impact on global health and highlighted the importance of international cooperation to effectively combat SARS-CoV-2. Since the discovery and publication of the virus’s genome in January 2020, scientists have rushed to develop vaccines, therapeutics and diagnostics on an unprecedented timescale. To date there are 80 vaccines in clinical trials and 70 more in clinical development, setting the stage for some of the fastest vaccine development and testing in modern history1. The vaccine technology platforms used by the most promising vaccine candidates range from viral vector–based and protein-based technologies to mRNA and lipid nanoparticle technology. Despite these impressive scientific achievements, barriers such as the vaccine cold chain and multiple forms of intellectual property (IP) protection stand in the way of equitable access and fair allocation.
Image: https://www.nature.com
