 The Annual BioHealth Capital Region Investment Conference is the premier destination for discovering emerging companies that are redefining what’s possible in life sciences.
The Annual BioHealth Capital Region Investment Conference is the premier destination for discovering emerging companies that are redefining what’s possible in life sciences.
Taking place on Thursday, September 25, the final day of BioHealth Capital Region Week 2025, the conference brings together startups, growth-stage companies, VCs, strategics, and innovation scouts for a dynamic day of curated pitches, one-on-one meetings, and strategic networking — all hosted at US Pharmacopeia in Rockville, Maryland.
For Investors:
This is where you’ll find what’s next. The conference showcases a vetted lineup of companies spanning therapeutics, diagnostics, digital health, AI-powered solutions, and other transformative technologies. You’ll hear live pitch presentations and quick-pitches on the main stage from top applicants, including standout participants from the previous day’s Crab Trap Competition.
Investors will schedule targeted meetings with presenting companies and engage with the region’s leading commercialization partners, accelerators, and research institutions. Whether you’re looking to make your next investment or build your pipeline, this is one of the best opportunities of the year to do it.
Investor registration is open now:
https://bit.ly/BHCRIC2025
For Startups and Entrepreneurs:
The Investment Conference is designed for companies ready to raise capital or build relationships with the investors who matter most in the BioHealth space. Whether you’re seeking Seed, Series A, or strategic partnerships, this event puts you directly in front of potential funders and collaborators.

 The Accelerate Investor Conference is a venture investor and startup conference and early stage business competition that showcases our region as a powerhouse for innovation and business opportunity.
The Accelerate Investor Conference is a venture investor and startup conference and early stage business competition that showcases our region as a powerhouse for innovation and business opportunity.


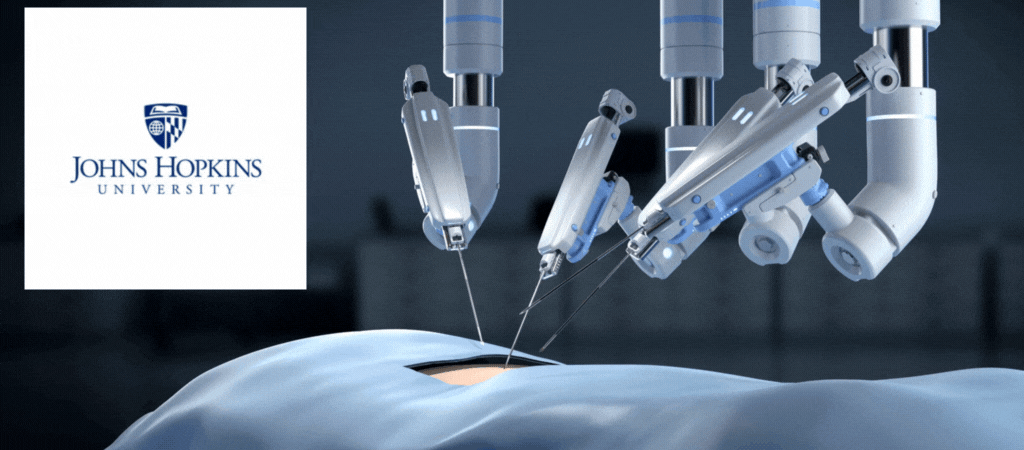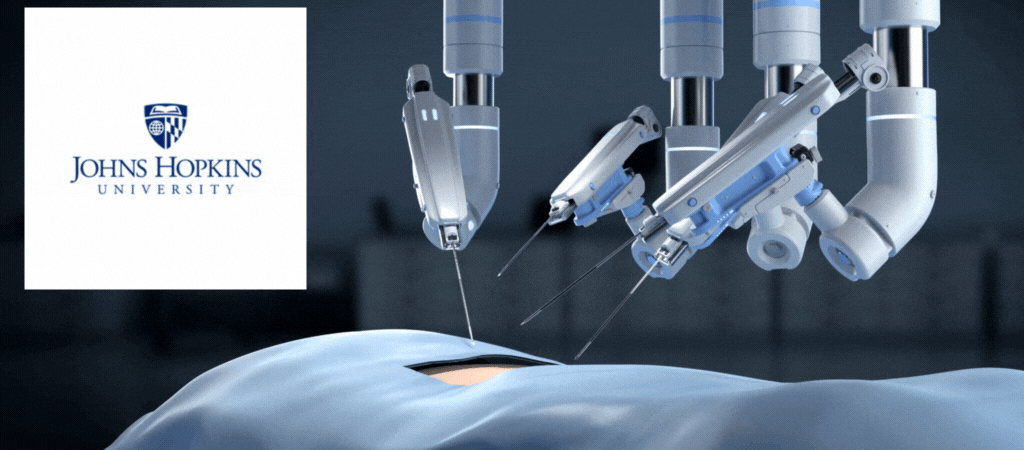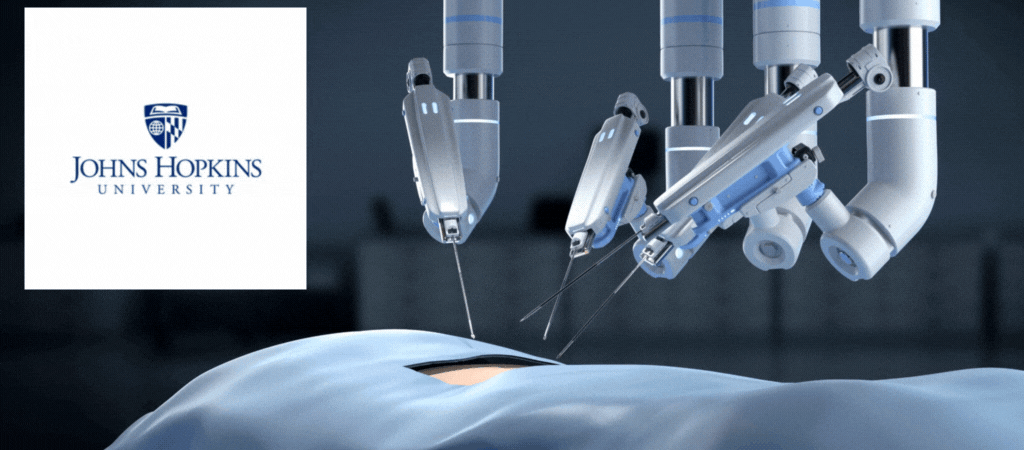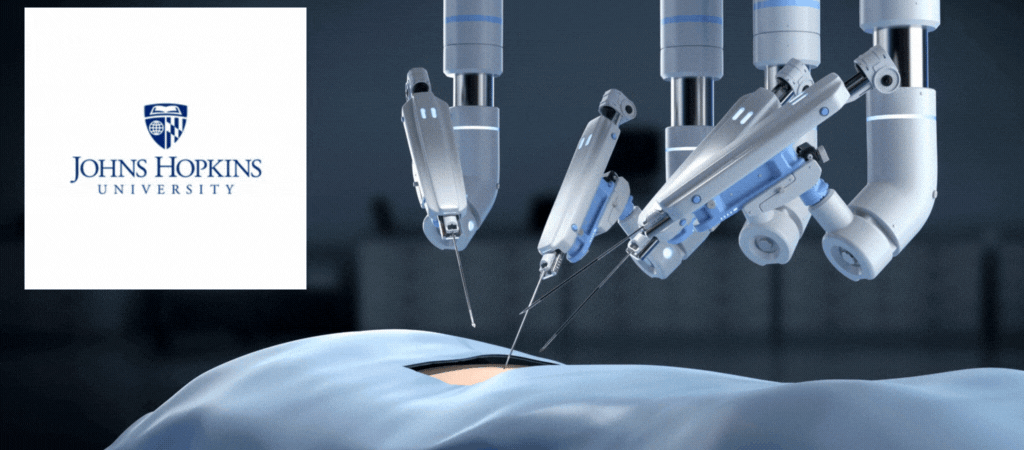
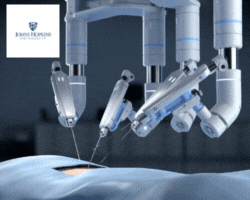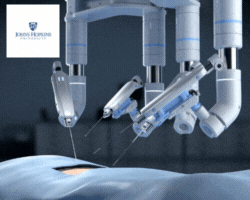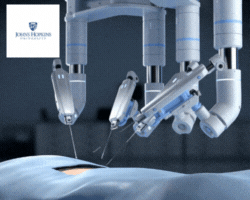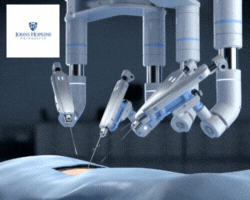 by Jill Rosen – A robot trained on videos of surgeries performed a lengthy phase of a gallbladder removal without human help. The robot operated for the first time on a lifelike patient, and during the operation, responded to and learned from voice commands from the team—like a novice surgeon working with a mentor.
by Jill Rosen – A robot trained on videos of surgeries performed a lengthy phase of a gallbladder removal without human help. The robot operated for the first time on a lifelike patient, and during the operation, responded to and learned from voice commands from the team—like a novice surgeon working with a mentor.
 Getting a biotech company off the ground takes more than science; it takes strategy, funding, and the right network.
Getting a biotech company off the ground takes more than science; it takes strategy, funding, and the right network. 
 GAITHERSBURG, Md., July 08, 2025 (GLOBE NEWSWIRE) — Emergent BioSolutions Inc. (NYSE: EBS) today announced a contract modification has been secured to deliver CNJ-016® [Vaccinia Immune Globulin Intravenous (Human)] (VIGIV) to the Administration for Strategic Preparedness and Response (ASPR), part of the U. S. Department of Health and Human Services (HHS), for smallpox preparedness. ASPR exercised an option from its existing 10-year contract (75A50119C00037) for additional doses of VIGIV, a treatment for complications due to smallpox vaccination.
GAITHERSBURG, Md., July 08, 2025 (GLOBE NEWSWIRE) — Emergent BioSolutions Inc. (NYSE: EBS) today announced a contract modification has been secured to deliver CNJ-016® [Vaccinia Immune Globulin Intravenous (Human)] (VIGIV) to the Administration for Strategic Preparedness and Response (ASPR), part of the U. S. Department of Health and Human Services (HHS), for smallpox preparedness. ASPR exercised an option from its existing 10-year contract (75A50119C00037) for additional doses of VIGIV, a treatment for complications due to smallpox vaccination.
 The Annual BioHealth Capital Region Investment Conference is the premier destination for discovering emerging companies that are redefining what’s possible in life sciences.
The Annual BioHealth Capital Region Investment Conference is the premier destination for discovering emerging companies that are redefining what’s possible in life sciences.
 ROCKVILLE, Md.–(BUSINESS WIRE)–The Maryland Tech Council (MTC), the largest technology and life sciences trade association in the state, today announced the launch of the Rural Technology Network, a new initiative to accelerate growth in rural Maryland’s technology ecosystem.
ROCKVILLE, Md.–(BUSINESS WIRE)–The Maryland Tech Council (MTC), the largest technology and life sciences trade association in the state, today announced the launch of the Rural Technology Network, a new initiative to accelerate growth in rural Maryland’s technology ecosystem.
 In this episode of BioTalk, Rich Bendis welcomes Dr. Helen Sabzevari, President and CEO of Precigen, to discuss the company’s cutting-edge science in gene and cell therapy. Dr. Sabzevari shares how Precigen’s unique AdenoVerse® platform has powered the development of PRGN-2012, a potential first-in-class therapeutic currently under FDA priority review for the treatment of adults with recurrent respiratory papillomatosis (RRP), a rare and devastating disease. She also highlights advances across Precigen’s broader pipeline in immuno-oncology and autoimmune disease and reflects on how Maryland’s BioHealth Capital Region has supported the company’s innovation and growth.
In this episode of BioTalk, Rich Bendis welcomes Dr. Helen Sabzevari, President and CEO of Precigen, to discuss the company’s cutting-edge science in gene and cell therapy. Dr. Sabzevari shares how Precigen’s unique AdenoVerse® platform has powered the development of PRGN-2012, a potential first-in-class therapeutic currently under FDA priority review for the treatment of adults with recurrent respiratory papillomatosis (RRP), a rare and devastating disease. She also highlights advances across Precigen’s broader pipeline in immuno-oncology and autoimmune disease and reflects on how Maryland’s BioHealth Capital Region has supported the company’s innovation and growth.