The U.S. Department of Health and Human Services, Biomedical Advanced Research and Development Authority (BARDA) has entered into a 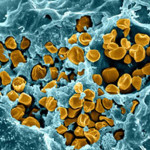 collaborative agreement with Glaxo Smith Kline (GSK) to evaluate the efficacy and safety of the company’s portfolio of clinical stage antibacterial assets for treating hospital and biothreat infections.
collaborative agreement with Glaxo Smith Kline (GSK) to evaluate the efficacy and safety of the company’s portfolio of clinical stage antibacterial assets for treating hospital and biothreat infections.
The contract is unique in that it is the first in which BARDA has taken a portfolio approach to funding drug development with industry.
