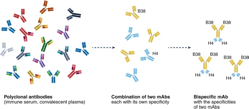
Human monoclonal antibodies (mAbs) are being used in the clinic to prevent or treat SARS-CoV-2 infection, and experience thus far suggests that combinations of antibodies are potentially able to sustain broad recognition of viral variants as they arise, but developing, testing and approving mAb combination products is complex. One approach to reducing the logistical challenges of combination products is the development of bispecific antibodies (bsAbs) (Fig. 1). In this issue of Nature Immunology, Li et al. report the development and testing of an engineered bispecific human mAb against SARS-CoV-21.
Image: https://www.nature.com
