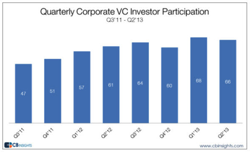

Corporate venture capital may be what fills in the large gaps VC has left behind, with CVC deals about 60 percent larger than VC deals on the whole.Healthcare corporate venture capital deals are up nearly 40 percent compared to Q1 and funding is up 34 percent, according to a report from CB Insights.
There were 126 CVC deals in Q2, ringing in at a combined value of $1.7 billion. Of these deals, 40 percent were at Seed or Series A-level, meaning CVCs may be willing to take risks VCs aren’t any more. According to the report, CVCs may help companies escape the Series A crunch: “One of every 4 CVC deals in Q2’13 were Series A deals ’ this represented an 800 basis point increase in Series A deal share vs. Q2’12.”