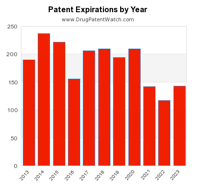

This live infographic dynamically shows the number of pending patent expirations by year for the next ten years.
For more infographics, see the DrugPatentWatch Pharmaceutical Innovation Infographics.
Holding place for old articles.


This live infographic dynamically shows the number of pending patent expirations by year for the next ten years.
For more infographics, see the DrugPatentWatch Pharmaceutical Innovation Infographics.


GlaxoSmithKline encountered some stiff industry headwinds when it pledged to open up its data vault to outside investigators. But as of today it has a high-profile convert on its side. The biopharma giant Roche ($RHHBY) has agreed to follow in GSK’s ($GSK) footsteps, saying that it will work with an independent group which will be charged with sorting out and approving requests for access to anonymized clinical trial data for all approved products. If regulators can’t provide the data, says Roche, then the company will make it available.
“We understand and support calls for our industry to be more transparent about clinical trial data with the aim of meeting the best interests of patients and medicine,” said Daniel O’Day, chief operating officer of Roche Pharma. “At the same time, we firmly believe that health authorities need to remain the gatekeeper for drug assessment and approval. We believe we have found a way in which patient data can be provided to third party researchers in a legitimate environment that ensures patient confidentiality and avoids the risk of publishing misleading results or giving rise to public health scares and consequences.”


Government contractors across the U.S. have found themselves in a holding pattern over the past few years, adapting to constant delays and waiting for economic uncertainty to be resolved. Between continuing resolutions, the debt ceiling crisis, and the threat of sequestration, government contracting companies have struggled to find new ways to be successful in the face of change.
According to a recent white paper by the Professional Services Council, “between fiscal years 2011 and 2012, federal spending on service contracts dropped more than $5 billion, which translates directly into the elimination of tens of thousands of contractor positions across the nation.” This, combined with a rise in the award of lowest-priced technically acceptable contracts, the delay in the award of new contacts and shorter term task orders under existing contract vehicles are all signs that a storm is brewing. Increased audits and investigations into contracting fraud waste and abuse, the increase in more regulations and compliance issues, and the Federal Strategic Sourcing initiative are clear evidence of this prevailing wind.


Johns Hopkins’ John Wong, Ph.D., has won a BioMaryland LIFE Award, and Ronald Berger, M.D., Ph.D., and Hien Nguyen, M.D., were awarded funds from the Abell Foundation, the researchers learned last week. Each of the winners will receive $50,000 to help develop their discoveries for clinical use.
The prizes were awarded as part of the annual Joint Meeting of the Johns Hopkins Alliance for Science and Technology Development and the University of Maryland, Baltimore Commercial Advisory Board on Feb. 19. The meeting was attended by more than 150 venture capitalists, seasoned biotech entrepreneurs and business development executives from the biopharma industry. Judging committees evaluated presentations from two dozen university researchers before selecting the winners. The aim of the awards is to speed the translation of promising research into commercial application.


New technology from the University of Maryland (UM) could potentially provide a five-minute diagnostic test and a vaccine for tough-to-treat Staphylococcus aureus infections, including the antibiotic-resistant MRSA, often called a “super bug,” says inventor Mark Shirtliff, PhD, an associate professor at the UM School of Dentistry in Baltimore.
Shirtliff is the winner of the 2013 BioMaryland LIFE (Leading Innovative Faculty Entrepreneurs) Prize for the most promising technology from the University as awarded by a judging panel at the annual joint meeting of the UM Baltimore Commercial Advisory Board and the Johns Hopkins University (JHU) Alliance for Science and Technology Development.
![]()
The Tech Council of Maryland (TCM), Maryland’s largest technology trade association with more than 400 life science and technology members employing more than 200,000 in the region, will honor Dr. James Barrett, a general partner in venture capital firm New Enterprise Associates (NEA), with its third annual Lifetime Achievement Award. Barrett will be presented the award at TCM’s Lifetime Achievement Gala, which is taking place March 6 at the Bethesda North Marriott Hotel and Conference Center.
The TCM Lifetime Achievement Award is given each year to a local individual who has gone above and beyond to serve the community at large over the course of his or her career. Recipients display commitment and leadership both in the field and within their company, fostering new ideas and encouraging creativity. The recipient also demonstrates generosity and compassion, making sure their work benefits others.


University of Maryland, College Park and two other East Coast schools will share $3.75 million from the National Science Foundation to develop a regional hub for turning university research into marketable products and services.
University of Maryland will be working with George Washington University and Virginia Tech to create an Innovation Corps for the mid-Atlantic region. The initiative will aim to draw out the best research ideas from students and faculty members and bring them to the commercial market.


McLean-based Science Applications International Corp., which announced plans in August to split off part of its operations into a separate publicly traded company, has decided on a name for the new business.
The spinoff, which will focus on national security, health and engineering, will be called Leidos.
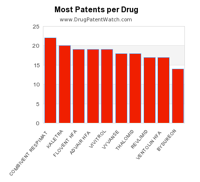

This chart shows the pharmaceutical drugs with the most patents still in force.
The drugs with the most active patents are COMBIVENT RESPIMAT, KALETRA, ADVAIR HFA,FLOVENT HFA, VIVITROL, THALOMID, VYVANSE, REVLIMID, VENTOLIN HFA, andCHILDREN’S ALLEGRA ALLERGY.


The Association of University Research Parks (AURP), the world’s leading network of university research, science and technology park professionals, invites you to share your knowledge, expertise and experience by presenting at the 2013 International Conference in Philadelphia, Pennsylvania. This year’s theme is Inventing the Future.
Proven engines for economic growth and development, university research parks influence their communities in significant ways. AURP’s 2013 annual conference, hosted by The University City Science Center, will feature experts who will examine university research park best practices and the strategies which will develop a knowledge-based economy by increasing ties between university, research parks, government, and industry partners.
Interesting approaches and creative solutions to challenges surrounding this topic are sought for presentations.