
 AstraZeneca intends to build a $1.5 billion manufacturing facility in Singapore for antibody drug conjugates (ADCs), enhancing global supply of its ADC portfolio. ADCs are next-generation treatments that deliver highly potent cancer-killing agents directly to cancer cells through a targeted antibody.
AstraZeneca intends to build a $1.5 billion manufacturing facility in Singapore for antibody drug conjugates (ADCs), enhancing global supply of its ADC portfolio. ADCs are next-generation treatments that deliver highly potent cancer-killing agents directly to cancer cells through a targeted antibody.
The planned greenfield facility, supported by the Singapore Economic Development Board (EDB), will be AstraZeneca’s first end-to-end ADC production site, fully incorporating all steps of the manufacturing process at a commercial scale. Manufacturing of ADCs is a multi-step process that comprises antibody production, synthesis of chemotherapy drug and linker, conjugation of drug-linker to the antibody, and filling of the completed ADC substance.

 Did you know? Each year, the federal government dedicates around $3.6 billion towards SBIR and STTR programs, resulting in awards to over 5,000 innovative projects!
Did you know? Each year, the federal government dedicates around $3.6 billion towards SBIR and STTR programs, resulting in awards to over 5,000 innovative projects!
The next deadline for NIH SBIR/STTR proposals is September 5th. As an innovation intermediary, BioHealth Innovation (BHI) is here to help you secure federal funding.
If you’re seeking assistance, don’t hesitate to reach out to Jon Nelson at JNelson@BioHealthInnovation.org. Let’s innovate together!

 At BioHealth Innovation Inc. (BHI), we are happy to celebrate the achievements of our partner company, miRecule, which has recently been named a winner at the INVEST Pitch Perfect competition. miRecule’s innovative approach to RNA therapies represents a significant advance in the treatment of cancer and muscular dystrophy. By leveraging genomic data to design highly specific therapeutics, miRecule stands at the forefront of personalized medicine, underscoring our commitment to supporting ground-breaking healthcare solutions.
At BioHealth Innovation Inc. (BHI), we are happy to celebrate the achievements of our partner company, miRecule, which has recently been named a winner at the INVEST Pitch Perfect competition. miRecule’s innovative approach to RNA therapies represents a significant advance in the treatment of cancer and muscular dystrophy. By leveraging genomic data to design highly specific therapeutics, miRecule stands at the forefront of personalized medicine, underscoring our commitment to supporting ground-breaking healthcare solutions.
This recognition is not just a win for miRecule; it’s a testament to the power of strategic partnerships and the impact of innovative biotechnological advances in addressing complex health challenges. BHI is proud to be associated with pioneers like miRecule, as we continue to foster a thriving ecosystem that brings revolutionary treatments from the lab to the patients who need them most.
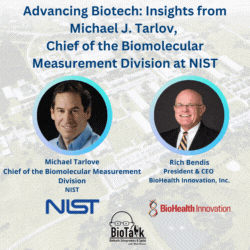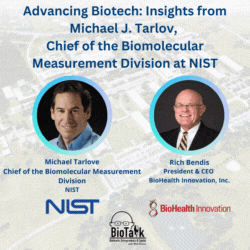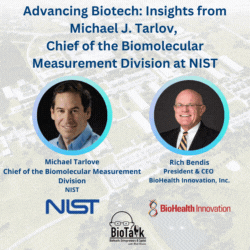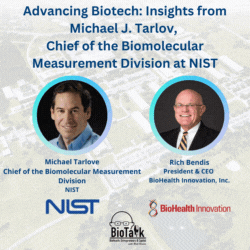
 Join us for an enlightening episode of BioTalk with Rich Bendis, featuring Michael J. Tarlov, Chief of the Biomolecular Measurement Division at the Material Measurement Laboratory, NIST. With a distinguished career in analytical chemistry and extensive experience in biochemical sensing and bioprocess measurements, Michael leads critical initiatives to advance biotechnology and healthcare.
Join us for an enlightening episode of BioTalk with Rich Bendis, featuring Michael J. Tarlov, Chief of the Biomolecular Measurement Division at the Material Measurement Laboratory, NIST. With a distinguished career in analytical chemistry and extensive experience in biochemical sensing and bioprocess measurements, Michael leads critical initiatives to advance biotechnology and healthcare.
In this episode, we delve into the pivotal role of NIST as a non-regulatory agency within the Department of Commerce, highlighting its profound impact on measurement science, gene therapy, gene editing, synthetic biology, and regenerative medicine. Michael shares insights into NIST’s collaborations, including local companies like MedImmune/AstraZeneca and Novavax, and the importance of partnerships with academic institutions like the University of Maryland in driving local biotech advancements.
Listen now via:
- Apple: https://apple.co/45acj2V
- Spotify: https://spoti.fi/3KpFmGj
- Amazon Music: https://amzn.to/3yL7oJK
- TuneIn: https://bit.ly/45bPT1s

 COLUMBIA, Md., (May 29, 2024) — TEDCO, Maryland’s economic engine for technology companies, unveiled the recently completed “Cybersecurity Workforce Analysis and Study” (“Study”). The Study was commissioned by TEDCO to better understand the current status of Maryland’s cybersecurity workforce pipeline. This knowledge will be used to guide the Cyber Maryland Program (“Program”) and its 18-member board of directors as they develop a statewide cybersecurity workforce development strategic plan.
COLUMBIA, Md., (May 29, 2024) — TEDCO, Maryland’s economic engine for technology companies, unveiled the recently completed “Cybersecurity Workforce Analysis and Study” (“Study”). The Study was commissioned by TEDCO to better understand the current status of Maryland’s cybersecurity workforce pipeline. This knowledge will be used to guide the Cyber Maryland Program (“Program”) and its 18-member board of directors as they develop a statewide cybersecurity workforce development strategic plan.
“The Study confirms what businesses and government agencies in Maryland already know: demand for skilled cybersecurity professionals—who protect our communities from criminals threatening our way of life—is incredibly nuanced. Thankfully, there is support and momentum amongst key stakeholders to bridge our talent gap,” said Roger Austin, chair of the Cyber Maryland board of directors. “My fellow board members and I are committed to address this critical need by developing innovative solutions to overcome our cyber skills talent gaps and provide good, paying jobs for our state’s residents.”

 COLUMBIA, Md. (May 30, 2024) – The Maryland Stem Cell Research Commission (“the Commission”) announces the release of Requests for Applications (RFAs) for the Maryland Stem Cell Research Fund’s (MSCRF) first funding cycle of fiscal year 2025 (FY25).
COLUMBIA, Md. (May 30, 2024) – The Maryland Stem Cell Research Commission (“the Commission”) announces the release of Requests for Applications (RFAs) for the Maryland Stem Cell Research Fund’s (MSCRF) first funding cycle of fiscal year 2025 (FY25).
RFAs for this initial cycle are now available for five grant programs: Manufacturing Assistance, Clinical, Validation, Commercialization, and Launch. All applications are due by July 9, 2024.
Along with this announcement comes the news that the Commission is also extending grant funding to businesses outside of Maryland for the Commercialization grant program, provided the research activities are conducted within the State. The Clinical grant program is available to entities with at least one clinical site in Maryland.

 GAITHERSBURG, Md., May 29, 2024 (GLOBE NEWSWIRE) — Today, Georgiamune Inc., a privately held, clinical-stage biotechnology company, announced the clearance of its second Investigational New Drug (IND) application by the U.S. Food and Drug Administration (FDA) for GIM-531, a first-in-class oral treatment that selectively targets T-regulatory cells and spares other immune cells such as CD4 and CD8. The news comes just on the heels of the company’s first-in-class, dual-functioning antibody, GIM-122, progressing to clinical trial for human testing.
GAITHERSBURG, Md., May 29, 2024 (GLOBE NEWSWIRE) — Today, Georgiamune Inc., a privately held, clinical-stage biotechnology company, announced the clearance of its second Investigational New Drug (IND) application by the U.S. Food and Drug Administration (FDA) for GIM-531, a first-in-class oral treatment that selectively targets T-regulatory cells and spares other immune cells such as CD4 and CD8. The news comes just on the heels of the company’s first-in-class, dual-functioning antibody, GIM-122, progressing to clinical trial for human testing.

 BALTIMORE, MD, US, May 29, 2024 /EINPresswire.com/ — Linshom Medical, a Maryland-based startup developing a wearable respiratory monitoring system, announced today that it has been awarded a $100,000 grant from the Maryland Industrial Partnerships (MIPS) program to support a clinical study of its respiratory monitoring device.
BALTIMORE, MD, US, May 29, 2024 /EINPresswire.com/ — Linshom Medical, a Maryland-based startup developing a wearable respiratory monitoring system, announced today that it has been awarded a $100,000 grant from the Maryland Industrial Partnerships (MIPS) program to support a clinical study of its respiratory monitoring device.
The study, “Advanced Prediction of Respiratory Depression Episodes with Linshom Continuous Predictive Respiratory Monitoring (CPRM),” will evaluate the Linshom Medical device’s ability for early detection of patient respiratory decline in the Post Anesthesia Care Unit. Dr. Samuel M. Galvagno, Executive Vice Chair of Anesthesiology and Interim Chair of the Department of Anesthesiology, will lead this effort for UMSOM as the principal investigator.

 NEW YORK – AMPEL BioSolutions is recruiting individuals with systemic lupus erythematosus (SLE) into an observational study of its blood-based prognostic gene expression assay LuGene to demonstrate a correlation between test results and standard clinical evaluations.
NEW YORK – AMPEL BioSolutions is recruiting individuals with systemic lupus erythematosus (SLE) into an observational study of its blood-based prognostic gene expression assay LuGene to demonstrate a correlation between test results and standard clinical evaluations.
In addition, the company has been conducting a study to see whether different lupus subtypes as defined by its test correlate with therapeutic response.
These studies follow another one published in October in the journal Genome Medicine that showed evidence for eight transcriptionally defined SLE endotypes, or subgroups, and demonstrated LuGENE’s ability to predict patients at risk for episodes of worsening symptoms called flares with high sensitivity and specificity.

 MANASSAS, Va.–(BUSINESS WIRE)–ATCC, the world’s premier biological materials management and standards organization, today announced that it has been awarded a task order with a ceiling value of $15.5 million, inclusive of base and options, by the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health (NIH). Under this task order 75N93019D00002-75N93024F00001, ATCC will develop well-characterized challenge material (WCCM) for the In Vitro Assessment for Antimicrobial Activity (IVAAA) program. This program aims to evaluate candidate countermeasures against microbial pathogens and vectors, including those derived from clinical specimens. The assessments are conducted in vitro and focus on antiviral screening assays for biosafety level (BSL)-3/4 viruses relevant to human diseases, as well as other rapidly emerging viruses that employ similar assay methodologies.
MANASSAS, Va.–(BUSINESS WIRE)–ATCC, the world’s premier biological materials management and standards organization, today announced that it has been awarded a task order with a ceiling value of $15.5 million, inclusive of base and options, by the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health (NIH). Under this task order 75N93019D00002-75N93024F00001, ATCC will develop well-characterized challenge material (WCCM) for the In Vitro Assessment for Antimicrobial Activity (IVAAA) program. This program aims to evaluate candidate countermeasures against microbial pathogens and vectors, including those derived from clinical specimens. The assessments are conducted in vitro and focus on antiviral screening assays for biosafety level (BSL)-3/4 viruses relevant to human diseases, as well as other rapidly emerging viruses that employ similar assay methodologies.
Due to the rise in countermeasures requiring the use of well-characterized viral stocks for animal studies and human clinical trials, researchers are examining the potential impact of cell substrates on the genetic stability and quality of progeny virus following serial propagation. In mammalian cells, serially propagating viruses can lead to rapid increases in genetic variants and instability, which have been shown to impact the interpretation of data from animal studies as well as from clinical trials. Ensuring the genomic homogeneity of WCCM, as well as improving the production capacity of WCCM used in drug screening studies, is essential for therapeutic drug development.
