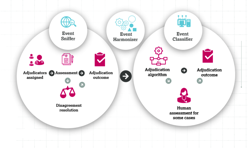
Automating adjudication of events in cardiovascular trials Clinical trials for cardiovascular (CV) disease are time consuming and expensive to run, often requiring large patient populations to meet the statistical requirements needed to demonstrate efficacy.1 These trials require robust data packages to achieve market approvals and registrations in order to get essential new medicines to patients.
Image: https://www.astrazeneca.com
