 Novel Nanotechnology Supercharges Immune System Molecules to Fight Cancer
Novel Nanotechnology Supercharges Immune System Molecules to Fight Cancer
Researchers from Cytimmune Sciences presented new data at a cancer therapeutics conference in Boston, MA demonstrating the potential of their nanoparticle-based platform to supercharge synergistic immune system stimulators called cytokines.
SUMMARY OF DATA PRESENTED
- Data focused on industry leading, first-in-class, multi-cytokine immunotherapies
- CYT-IFNg-TNFa (A dual cytokine immunotherapy nanomedicine)
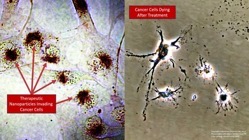
- 100x increase in direct cancer cell killing potency vs native cytokines
- 30x increase in cytokine accumulation in tumor tissue
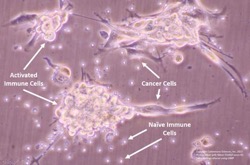
- Induction of a cancer specific immune response after treatment
- Other novel cytokine-based immunotherapies
- Introduction of nanomedicines including combinations of IL-12, IL-2, IL-15 & IL-1b
- 175x increase in potency of IL-12 presented on nanoparticles vs. native IL-12
- CYT-6091 (high dose TNF alpha)
- Unpublished preclinical data showing CYT-6091 induces cytotoxic T-Cell tumor infiltration
- Phase 1 clinical safety data and preclinical mechanism of action data
ROCKVILLE, Md., Aug. 23, 2022 /PRNewswire/ — Researcher from Cytimmune Sciences, a clinical-stage biotechnology company, presented new data on the company’s novel cancer nanomedicines at the Cytokine Drug Development Conference held in Boston, Massachusetts on July 26-28. David Oarr, Cytimmune’s president, participated in the events opening panel to discuss the current clinical status and future anticipated progress in the use of cytokines in the treatment of cancer. Giulio Paciotti, PhD, Cytimmune’s chief science officer, was a featured speaker at the event and presented data on the company’s novel cytokine-based nanomedicines, which have been designed to make these promising immune stimulating molecules safer and more effective.
Cytokines are immune system signaling molecules that have enormous clinical potential including the ability to destroy critical tumor support structures such as blood vessels, drive a multi-faceted anti-cancer immune response, and initiate targeted killing of cancer cells.
David Oarr described the challenges historically associated with cytokine delivery.
“Clinical use of this class of immune system molecules has been hampered by high levels of off-target toxicity, an inability to deliver multiple cytokines in combination, and very short half-lives. Cytimmune is focused on designing therapies that overcome these challenges.”
Dr. Paciotti presented a combination of preclinical and clinical data on the company’s nanomedicine assets to demonstrate that Cytimmune’s nanoparticle platform may provide a viable clinical solution to the toxicity and short half-life challenges associated with most cytokines. Additional data were presented to illustrate Cytimmune’s unique approach to packaging combinations of high doses of multiple cytokines on a single nanoparticle. Dr. Paciotti explained the significance of this technology:
“Combination delivery of cytokines is essential to maximizing the efficacy of these molecules against solid tumor cancers. We believe our data clearly show that using our nanoparticle platform to simultaneously deliver synergistic cytokines such as Interferon gamma and Tumer Necrosis Factor alpha, or Interleukins 2 and 12, can result in an increase in anti-cancer effect of more than 100-fold. Additionally, our preclinical data show that even individual cytokines delivered on our nanoparticle platform are vastly more potent than native cytokines delivered at the same concentration. I believe supercharged cytokine combinations have the potential to be a game-changer immunotherapy in the treatment of solid tumor cancers.”
ABOUT CYTIMMUNE
Cytimmune is a phase 1b/2 ready oncology company developing multiple assets to treat solid tumor cancers. The company’s lead asset, CYT-6091, is a novel cancer nanomedicine that destroys tumor blood vessels within hours of administration and recruits cytotoxic T-Cells into solid tumors. The U.S. National Cancer Institute (NCI) has signed a clinical trial agreement with Cytimmune to conduct phase 1b and multiple phase 2 clinical trials in which CYT-6091 will be combined with paclitaxel to treat orphan endocrine cancers of the thyroid and pancreas.
BACKGROUND LINKS
http://cytimmune.com
https://cytokine-immunotherapies.com/
SAFE HARBOR STATEMENT
This press release includes forward-looking statements that may be identified by words such as “believe,” “expect,” “may,” “plan,” “potential,” “will,” “can,” “preliminary,” and similar expressions, and are based on management’s current beliefs and expectations. Forward-looking statements are subject to risks and uncertainties, and Cytimmune cautions against placing undue reliance on such statements. Actual results may differ from those set forth in the forward-looking statements. Any forward-looking statements speak only as of the date of this press release and the Company assumes no obligation to update any forward-looking statement.
Media Contact:
David Oarr
202-725-0477
342947@email4pr.com
SOURCE Cytimmune Sciences
