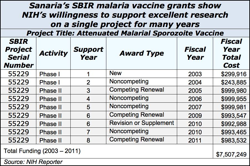
There’s a special place in NIH’s heart for SBIR research to develop drugs, medical devices and other products that require FDA approval. For these capital intensive products where time horizons for market entry are long, many NIH institutes offer extra millions and extra years of SBIR grant support after Phase II ends.
For most institutes, SBIR Phase IIB Competing Renewal grants are the vehicle for giving extra money. At NCI and NHLBI, Bridge grants do the same thing.
