OCT 06, 2025 08:30 EST
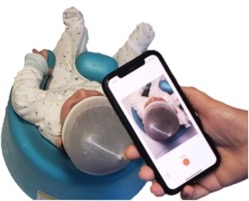
PediaMetrix received 510(k) clearance from the US Food and Drug Administration (FDA) for SoftSpotTM, a cross platform software solution for infant cranial evaluation at the point of care. The FDA clearance demonstrates SoftSpot’s safety and efficacy for infant cranial measurements using a smartphone.
“This is an important milestone for PediaMetrix and we see great potential in now bringing this innovative technology to pediatricians in the US and around the world”, said Fereshteh Aalamifar, PhD, President & CEO of PediaMetrix. Currently, pediatricians are not equipped with any tool for quantitative measurement of cranial shape and only perform visual assessment. They refer parents of children at risk to specialists for quantitative measurements, which can cause delays in the early detection and intervention. SoftSpot brings the power of a specialist to a pediatric office using a smartphone.
“Conditions with cranial deformity are on the rise and affect over 20% of newborns. These conditions, including deformational plagiocephaly and brachycephaly and craniosynostosis, cause an estimated $1B cost to the US healthcare.” said Aalamifar. Using computer vison, AI, and smartphones, SoftSpot will provide pediatric clinicians with the support required to early detect, monitor, and treat head deformities. Patients and their families will avoid intensive and expensive treatments.”
SoftSpot demonstrated over 93% correlation with the standard cranial measurements used for the clinical determination of deformational plagiocephaly and brachycephaly. The technology will be launched in the US during Q4 of 2021.
About PediaMetrix Inc.
Founded by alumni entrepreneurs and scientists from Johns Hopkins, NIH, Oxford, and Princeton, PediaMetrix’s mission is to bring AI and computer vision to pediatrics to lower the healthcare costs and improve outcomes. PediaMetrix’s lead product, SoftSpot, enables early detection and management of the most common conditions with head shape abnormalities in newborns. PediaMetrix is a Delaware company founded in 2018 and currently has 10 full-time and part-time employees. The main office is in Rockville, MD.
Read more about PediaMetrix and SoftSpot at www.pediametrix.com
For more information, please contact:
Dr. Fereshteh Aalamifar, Founder & CEO
Tel: +1 (240) 670-0171
Email: Fereshteh@pediametrix.com
Tags:
